Write4U
Valued Senior Member
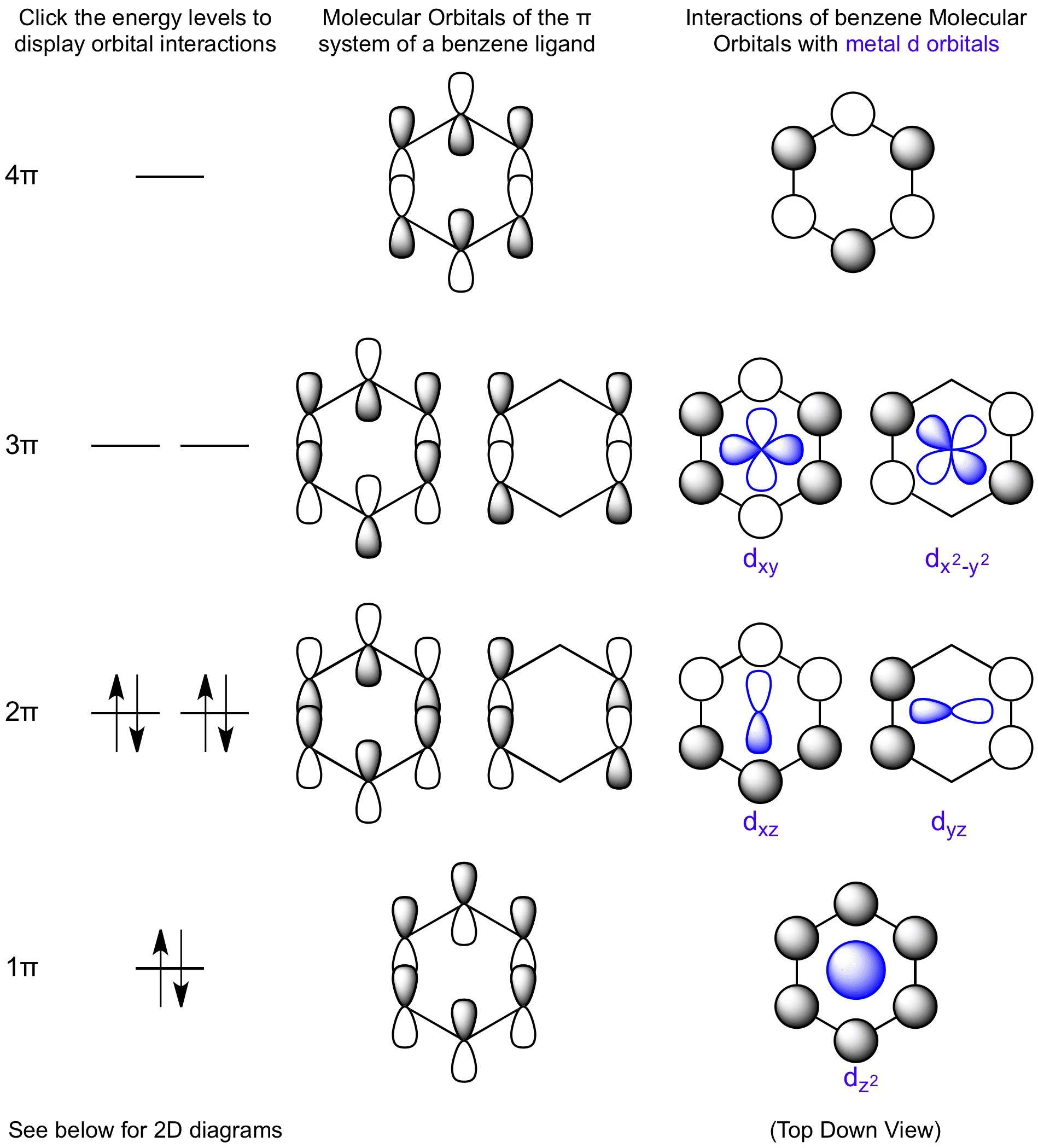
Clearing up a few outstanding issues about the importance of benzene rings in microtubules.
Consciousness, Cognition and the Neuronal Cytoskeleton – A New Paradigm Needed in Neuroscience
 Stuart Hameroff1,2,3*
Stuart Hameroff1,2,3*
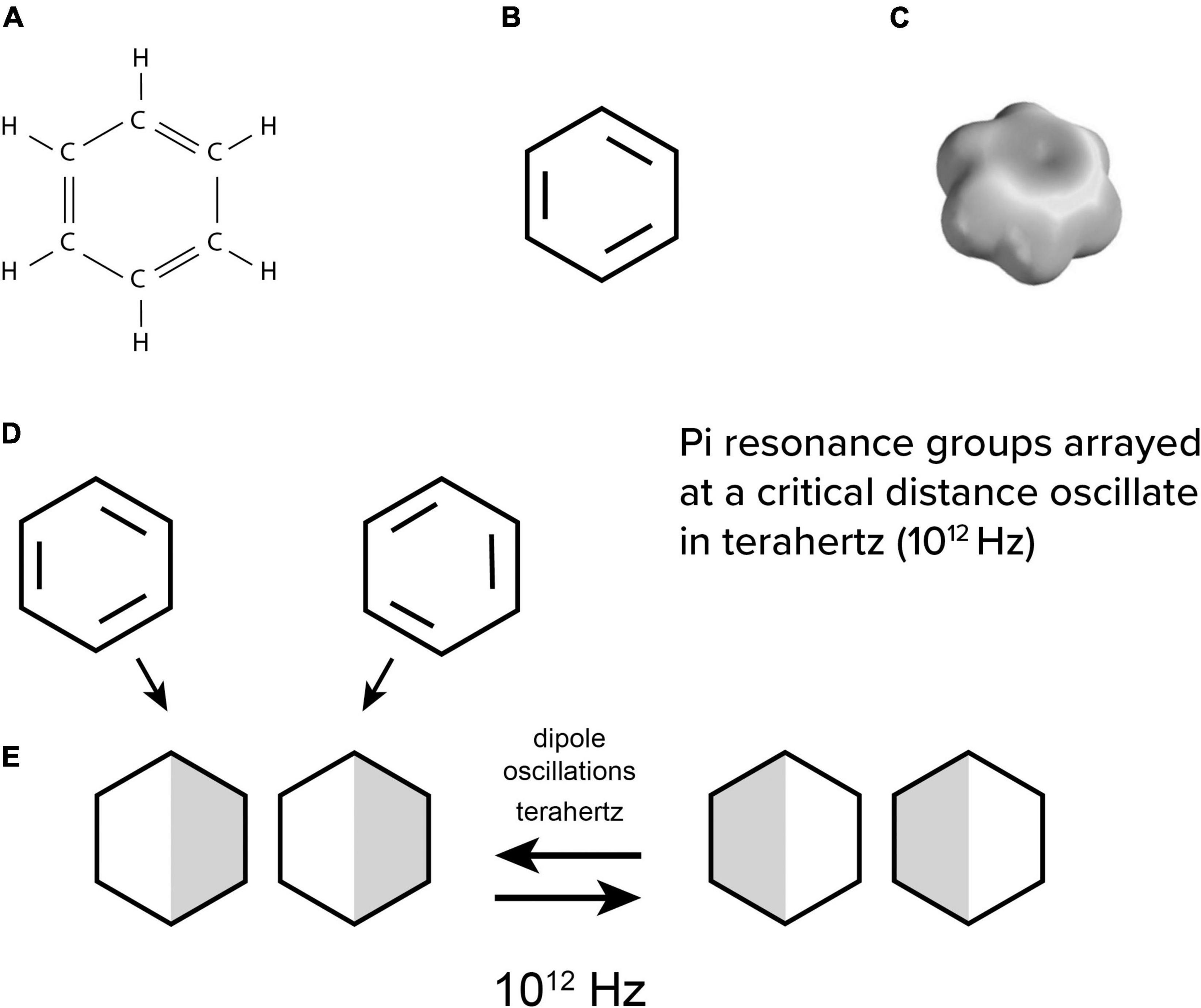
FIGURE 4. (A) chemical structure of benzene, (B) simplified with 3 extra electron bonds, and (C) with a delocalized electron cloud enveloping the ring. (D) individual benzene rings attract by induced dipoles which then (E) oscillate in terahertz.
https://www.frontiersin.org/articles/10.3389/fnmol.2022.869935/full
Does this clear up any confusion about the role played by benzene rings in the MT network?
Consciousness, Cognition and the Neuronal Cytoskeleton – A New Paradigm Needed in Neuroscience
 Stuart Hameroff1,2,3*
Stuart Hameroff1,2,3*- 1Department of Anesthesiology, The University of Arizona, Tucson, AZ, United States
- 2Department of Psychology, The University of Arizona, Tucson, AZ, United States
- 3Center for Consciousness Studies, The University of Arizona, Tucson, AZ, United States
Automata and computer systems generally utilize some type of periodic clocking mechanism to synchronize and update operations. In the microtubule automata simulations done by Rasmussen et al. (1990), collective coherent dipole oscillations among tubulins provided coherent time steps for synchronized updates, for example steps 1–4 in Figure 3 on the right. pThe oscillation updates result in propagating patterns of information, the biological mechanism for this clocking ascribed to “Fröhlich coherence.”
Biophysicist Herbert Fröhlich (1968, 1970, 1975) suggested that dipoles in non-polar intra-protein regions would oscillate due to the most basic structure in organic chemistry—the “pi electron resonance” benzene ring (Figure 4). Each of the hexagonal benzene’s 6 carbons has 4 electron bonds to share with 2 hydrogens and 2 neighbor carbons in a planar ring (Figures 4A,B).
Figure 4This leaves 3 extra “pi orbital” resonance electrons which are portrayed as double bonds resonating between different carbons. In molecular orbital theory, the pi electrons combine and “delocalize” in a space-filling electron cloud enveloping all 6 carbons (Figure 4C). Such clouds are chemically neutral and non-polar, but polarizable, leading to interesting quantum vibrational properties.

FIGURE 4. (A) chemical structure of benzene, (B) simplified with 3 extra electron bonds, and (C) with a delocalized electron cloud enveloping the ring. (D) individual benzene rings attract by induced dipoles which then (E) oscillate in terahertz.
https://www.frontiersin.org/articles/10.3389/fnmol.2022.869935/full
Does this clear up any confusion about the role played by benzene rings in the MT network?
Last edited: