Write4U
Valued Senior Member
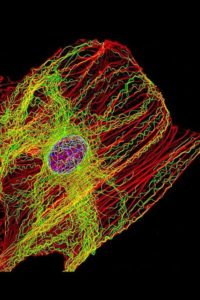
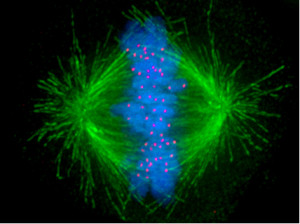
Yet another example of the compound functional value provided by the microtubule and related filamental networks and functions.
Microtubule-Based Mitochondrial Dynamics as a Valuable Therapeutic Target in Cancer
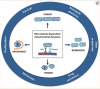
Figure 1
Microtubule-dependent mitochondrial dynamics: Through the balance between fusion/fission and biogenesis/mitophagy, mitochondrial dynamics represent a central process in the bioenergetic adaptation and metabolic plasticity of cancer cells. The balance between biogenesis and mitophagy regulates the number of mitochondria and their quality. The fusion process helps to increase mitochondrial metabolism and to limit mitophagy and apoptosis, while the fission process allows the spatial redistribution of mitochondria in areas of the cell with greater energy and metabolic needs, favoring cell spreading and metastases.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8616325/
Microtubule-Based Mitochondrial Dynamics as a Valuable Therapeutic Target in Cancer
Simple Summary
Mitochondria are well known for being the powerhouses of the cell—whether the cell is normal or cancerous. Moreover, they can move, split, fuse themselves, or be eliminated via mitophagy with the help of the interplay between motor proteins and the cell scaffold—especially microtubules. The relationship between mitochondria, microtubules, and motor proteins is altered in cancer, and targeting this molecular machinery can offer a novel weapon in its treatment. In this paper, we review and summarize the state of the art of this approach.
Abstract
Mitochondria constitute an ever-reorganizing dynamic network that plays a key role in several fundamental cellular functions, including the regulation of metabolism, energy production, calcium homeostasis, production of reactive oxygen species, and programmed cell death. Each of these activities can be found to be impaired in cancer cells. It has been reported that mitochondrial dynamics are actively involved in both tumorigenesis and metabolic plasticity, allowing cancer cells to adapt to unfavorable environmental conditions and, thus, contributing to tumor progression.
The mitochondrial dynamics include fusion, fragmentation, intracellular trafficking responsible for redistributing the organelle within the cell, biogenesis, and mitophagy. Although the mitochondrial dynamics are driven by the cytoskeleton—particularly by the microtubules and the microtubule-associated motor proteins dynein and kinesin—the molecular mechanisms regulating these complex processes are not yet fully understood.
1. IntroductionMore recently, an exchange of mitochondria between stromal and cancer cells has also been described. The advantage of mitochondrial transfer in tumor cells results in benefits to cell survival, proliferation, and spreading. Therefore, understanding the molecular mechanisms that regulate mitochondrial trafficking can potentially be important for identifying new molecular targets in cancer therapy to interfere specifically with tumor dissemination processes.
The cytoskeleton is a dynamic and interconnected network of filaments composed of structural and regulatory proteins that play a key role in all fundamental cellular processes, such as shape retention, motility, division, and intracellular transport of proteins and organelles [1,2]. Therefore, it is not surprising that alterations in cytoskeletal function can contribute to the onset and progression of cancer [3]. The three main types of filament that characterize the cytoskeleton are microfilaments, microtubules, and intermediate filaments [4]. Several ultrastructural analyses have shown that the cytoskeletal filaments interact directly or indirectly with the plasma membrane and various intracellular organelles [5].
Microtubules have a distinct polarity that is critical for their biological function. Tubulin polymerizes end-to-end; therefore, in an MT, one end will have the α-subunits (minus) exposed, while the other end will have the β-subunits (plus) exposed [7].
MTs are essential in many vital cellular processes, such as structural support, mitosis, chromosome segregation during meiosis, and intracellular transport of vesicles and organelles such as mitochondria [1,2,7]. In particular, to facilitate the movement of vesicles and mitochondria along their tracks, MTs recruit motor proteins via acetylation on lysine 40 of α-tubulin [8,9,10,11]. Microtubule-associated motor proteins include kinesin and dynein, which carry their cargo to the minus and plus ends of the microtubules, respectively [12,13]. In vitro studies have revealed that the loss of acetylated residues in MTs reduces the interaction of kinesin with tubulin, with a subsequent decrease in cell motility [10].
MTs have been an ideal target in antineoplastic therapy for many years, as they are the main components of the mitotic spindle. In addition, these filaments, together with motor proteins, play a fundamental role in the mitochondria’s structural and functional organization, including morphology, dynamics, motility, and distribution (Figure 1) [14].

Figure 1
Microtubule-dependent mitochondrial dynamics: Through the balance between fusion/fission and biogenesis/mitophagy, mitochondrial dynamics represent a central process in the bioenergetic adaptation and metabolic plasticity of cancer cells. The balance between biogenesis and mitophagy regulates the number of mitochondria and their quality. The fusion process helps to increase mitochondrial metabolism and to limit mitophagy and apoptosis, while the fission process allows the spatial redistribution of mitochondria in areas of the cell with greater energy and metabolic needs, favoring cell spreading and metastases.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8616325/
Last edited:


 S. Aryana Yousefzadeh
S. Aryana Yousefzadeh Germund Hesslow
Germund Hesslow Gleb P. Shumyatsky
Gleb P. Shumyatsky Warren H. Meck
Warren H. Meck